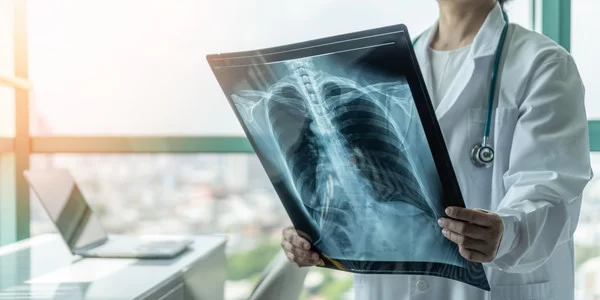
Strategic partnership in brain imaging and AI PARIS, MONTREAL, and QUEBEC CITY, Oct. 13, 2020 — Acquisition of TRUE POSITIVE MEDICAL DEVICES by QYNAPSE A strategic collaboration that covers 15 patents, including 9 issued in the U.S. and Canada A…
Life-changing medical innovations that makes the impossible possible
STOCKHOLM, Oct. 8, 2020 — Medical wire that stops the trembling caused by Parkinson’s disease. Precision tools that can manufacture tailored prosthetic limbs durable and flexible enough to race down the slopes. And 3D-printing technologies that can create scull and spinal implants in just a few hours. With new and…
Smartphone-based Solutions Boost the Global Infectious Disease Point-of-Care Testing Market at an Explosive CAGR of 70.2% in 2020
Digital POCT platforms will enable diagnostic testing beyond the clinic, transforming how assay results are gathered and analyzed, finds Frost & Sullivan SANTA CLARA, California, Oct. 2, 2020 — Frost & Sullivan’s recent analysis, Smartphone-based Solutions Spur the Global Infectious Disease Point-of-Care Testing Market, Forecast to 2024, finds that the outbreak…
Turning AI onto itself: AI algorithm detects when medical images will be difficult for radiologists or AI to make an effective diagnosis
SAN FRANCISCO, Sept. 30, 2020 — A new AI algorithm can identify when medical images are likely to be difficult for either a radiologist or AI to make an effective diagnosis. The algorithm can potentially be used to triage medical scans and highlight cases that warrant further in-depth clinical evaluation…
Infosys to Acquire Product Design and Development firm, Kaleidoscope Innovation
Expands engineering services portfolio by strengthening presence in Medical devices, Consumer and Industrial markets across US BENGALURU, India and CINCINNATI, Sept. 4, 2020 — Infosys (NYSE: INFY), a global leader in next-generation digital services and consulting, today announced a definitive agreement to acquire Kaleidoscope…
Jvion Lauded by Frost & Sullivan for Improving Patient Outcomes Using Its Clinical-AI CORE™ Intelligence Platform
Jvion’s prescriptive analytics approach identifies undetected risk and modifiable risk trajectories followed by recommendations for remedial actions SANTA CLARA, California, Sept. 1, 2020 — Based on its recent analysis of the North American prescriptive analytics market, Frost & Sullivan recognizes Jvion, Inc. with the 2020 North American Technology Innovation Leadership Award for…
G-ray Switzerland announces new CEO, closes successful funding round
NEUCHÂTEL, Switzerland, Aug. 31, 2020 — G-ray Switzerland, the medical imaging and industrial diagnosis start-up founded in 2014, has announced the appointment of Luis Pallares as Chief Executive Officer, as the company embarks on an accelerated growth drive and completes a successful funding round. Mr Pallares is focused on leading the transformation of…
Dymax Asia Pacific Pte Ltd Receives the Singapore SME 1000 & SME 1000 Award and Winner of D&B Business Eminence Award
TORRINGTON, Conn., Aug. 14, 2020 — Dymax Corporation, leading manufacturer of rapid light-curing materials and equipment, is pleased to announce that its Singapore subsidiary, Dymax Asia Pacific Pte Ltd., has received the Singapore 1000 & Singapore SME 1000 award in its annual ranking of best companies in 2020. And it…
Global Healthcare Interoperability Market to Witness Nearly Two-fold Growth by 2024
Data interoperability and data analytics are key contributors to global market revenue for healthcare interoperability, says Frost & Sullivan SANTA CLARA, California, July 27, 2020 — Frost & Sullivan’s recent analysis, Global Healthcare Interoperability Market, Forecast to 2024, contends that interoperability has become a critical consideration for all health IT…
FUJIFILM Sonosite Launches New Point-Of-Care Ultrasound System With Adaptable Form Factor, Embedded Workflow, And The Most Advanced Image Clarity Sonosite Has Ever Offered
BROOKVALE, New South Wales, July 24, 2020 — FUJIFILM Sonosite, Inc., specialists in developing cutting-edge point-of-care ultrasound (POCUS) solutions, and part of the larger Fujifilm Healthcare portfolio, has announced the launch of the new Sonosite PX ultrasound system. Sonosite PX is the next…