– Leading efficient care management for the elderly with unimpeded smartcare https://img.hankyung.com/pdsdata/pr.hankyung.com/uploads/2024/11/image01-1.png SEOUL, South Korea, Nov. 23, 2024 — JCF Technology is a startup that independently developed ‘MecKare’, a radar…
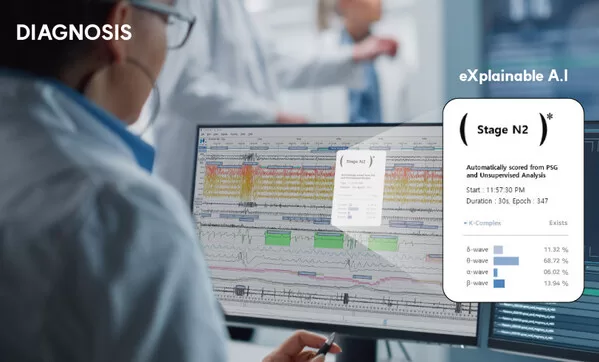
HoneyNaps actively supplies “AI solutions” to U.S. medical institutions
Rapid commercialization and delivery to the U.S. after FDA approval Aiming to achieve a 10% market share within three years Aggressive sales and expansion of distribution network through…
On-Device AI Healthcare Company Noul Announces 2 Clinical Performance Studies at Pan-African Malaria Conference
miLab showed almost equivalent to WHO-certified first-degree microscopy experts: Sensitivity of 94.4%, Specificity of 98.1% African authority on malaria diagnosis said…
TOPDON Unveils State-of-the-Art Thermal Imaging Camera with 9mm Adjustable Lens
ROCKAWAY, N.J., April 25, 2024 — TOPDON, the premier provider of cutting-edge technology and advanced tools for auto repair professionals and enthusiasts,…
PranaQ to Unveil Revolutionary Sleep Tech at CES 2024: Introducing TipTraQ, the AI Sleep Tracking and Improvement Solution
NEW YORK, Jan. 16, 2024 — PranaQ, the innovative sleep tech startup, has made waves at CES 2024 with the highly anticipated launch of its flagship product, TipTraQ. This…
Lunit Joins as an Associate Partner with the World Economic Forum
– Lunit joins as an Associate Partner of the World Economic Forum, propelling global expansion and collaboration in the realm of AI-driven…
Mediballoon’s Online Shop is now live, offering a range of medical balloons and tubing for sale. Mediballoon is your best partner for medical device development.
UNION CITY, Calif., Aug. 9, 2023 — Mediballoon, Inc., a Medeologix company established in 2016 specializing in medical balloon design, development, and scale-up production, is excited to announce the…
Lunit’s AI-Powered Lung Cancer Screening Solution Significantly Affects Radiologists’ Diagnostic Determination – Published in Radiology
– Recent study conducted by Seoul National University Hospital provides strong evidence that high-accuracy AI model improves radiologists’ chest X-ray analysis performance…
United Imaging Introduces Next-Generation PET/CT Systems and Integrated Molecular Technology Platform at SNMMI
With uMI Panorama™, United Imaging Continues to Push the Envelope on Whole-Body System Performance HOUSTON, June 26, 2023 — United Imaging, a global leader in manufacturing advanced medical imaging…
Jim Lim joins Zühlke Group as Head of Health & Medtech in Singapore
SINGAPORE, April 21, 2023 — Zühlke Group is pleased to announce the appointment of Jim Lim as Head of Market Unit (Health & Medtech) at Zühlke Asia. Based in Singapore, Jim…