- Rapid commercialization and delivery to the U.S. after FDA approval
- Aiming to achieve a 10% market share within three years
- Aggressive sales and expansion of distribution network through U.S. subsidiaries
BOSTON, Oct. 7, 2024 /PRNewswire/ — HoneyNaps announced on September 9th that it has officially begun exporting by delivering its artificial intelligence sleep disorder diagnostic software “SOMNUM” to two medical institutions in the U.S.
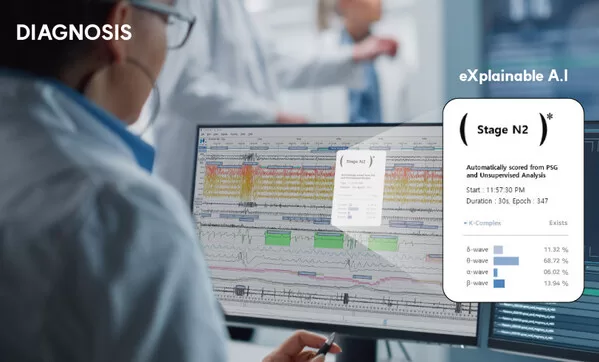
The SOMNUM solution supplied to the U.S. is the third globally to receive approval from the U.S. Food and Drug Administration(FDA) in 2023. It is not just a system for interpreting biometric signal images, but a deep learning-based AI interpretation system capable of real-time analysis of multi-channel/temporal and large-scale biometric signals, developed using the world’s first explainable medical AI (XAI, eXplainable AI) technology.
The SOMNUM solution will be introduced into research on sleep health for seniors and the verification of sleep improvement programs. HoneyNaps has signed a licensing agreement based on annual usage fees for the software.
HoneyNaps is currently strengthening its B2B business with major companies in South Korea while simultaneously focusing on local sales through its U.S. subsidiary in Boston and its West Coast dealer channels, expecting continuous growth in export volume.
A HoneyNaps representative said, “The U.S. is the largest sleep medicine market in the world and still holds significant growth potential. As we officially enter the U.S. market, we will solidify our business strategy to achieve a 10% market share within three years.”
For further information, please contact:
HoneyNaps USA, Inc.
Christine Kwon / Managing Director
Email: [email protected]
Address: #517, 5th Fl., 361 Newbury Street, Boston, MA 02115
Website: www.honeynaps.com