Highly complementary transaction strengthens BioAgilytix’s ability to support bioanalytical services across all major therapeutics in development; adds LC/MS/MS capabilities; and expands team of seasoned scientists DURHAM, N.C., Aug. 19, 2021 — BioAgilytix Labs, LLC (BioAgilytix), a leading global contract research laboratory focused on supporting its pharmaceutical and biotech…
Atonarp announces innovative new metrology platform Aston, aimed at increasing yield, throughput, and efficiency in semiconductor manufacturing processes
TOKYO, July 15, 2021 — Atonarp, a leading manufacturer of molecular sensing and diagnostics products for the semiconductor, healthcare, and pharma industries, today announced Aston, an innovative in-situ semiconductor metrology platform with an integrated plasma ionization source. Atonarp’s Aston…
Enamine implements CDD Vault to digitalize its integrated medicinal chemistry, ADME-PK and screening services
The company’s expansion in the biological assay space aided by the data platform SAN FRANCISCO and KYIV, Ukraine, June 10, 2021 — Enamine, a leading provider of R&D Services as well as Screening Compounds, Fragments, Building Blocks and specialized libraries for Drug Discovery, announced…
Insilico enters into a collaboration with APRINOIA to apply novel generative AI-powered system to discover novel compounds for neurodegenerative diseases
TAIPEI, Taiwan, Dec. 30, 2020 — Insilico Medicine is pleased to announce that it has entered into a research collaboration with APRINOIA Therapeutics to utilize Insilico’s novel generative artificial intelligence (AI) technology to accelerate the discovery of next generation compounds targeting abnormal proteins in brain associated with neurodegenerative diseases. …
Tony Blair Institute and Oracle Launch Africa Vaccine Management in the Cloud
The Tony Blair Institute for Global Change and Oracle partner with African governments to manage large-scale vaccination programs in the cloud – over 73,000 people vaccinated and registered in the first 8 days in Ghana LONDON and REDWOOD SHORES, Calif., Nov. 23, 2020…
The fourth CIIE begins taking stall reservations from exhibitors
SHANGHAI, Nov. 13, 2020 — Some exhibitors planning to attend the fourth China International Import Expo (CIIE) next year started to reserve their stalls on Nov 8. This is the first time that the CIIE has offered such an option. Five dairy companies…
AlpVision to offer free of charge security feature to protect COVID-19 relevant medicines against counterfeiting
VEVEY, Switzerland, Nov. 13, 2020 — On November 13th, 2020, AlpVision is launching the "AlpVision COVID-19 Initiative" helping pharmaceutical companies to protect COVID-19 relevant medicines against counterfeiting. Authentication using smartphones COVID-19 has caused not only a worldwide health crisis,…
Clarivate Expands International Real-World Data Offering with Addition of Techtrials Brazilian Dataset
Partnership to provide life science, healthcare and research professionals with comprehensive RWD capturing approximately 80% of the Brazilian population LONDON, Nov. 2, 2020 — Clarivate Plc (NYSE:CCC), a global leader in providing trusted information and insights to accelerate the pace of innovation, today announced the…
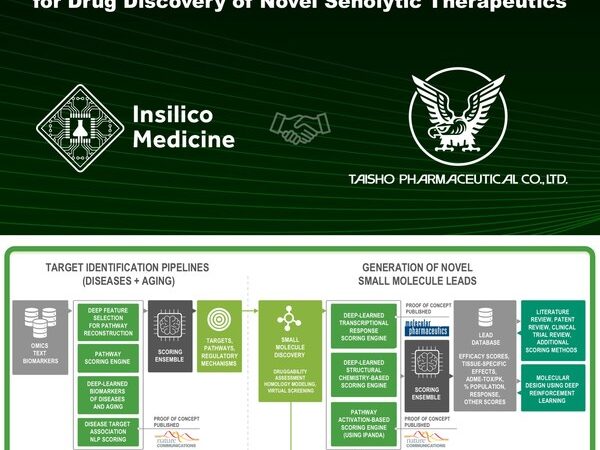
Insilico partners with Taisho on end-to-end AI-powered senolytic drug discovery
HONG KONG, Oct. 15, 2020 — Insilico Medicine announced today that Taisho Pharmaceutical Co., Ltd. and Insilico have entered into a research collaboration to identify novel therapeutics against aging. Insilico Medicine will utilize both the target discovery and generative chemistry parts of its Pharma.AI platform in this collaboration. It will use…
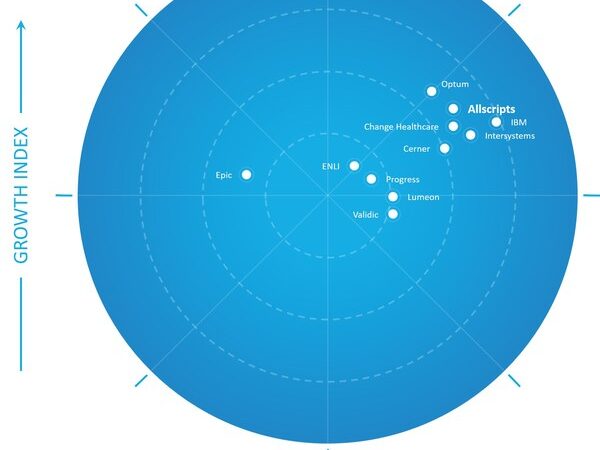
Frost & Sullivan Recognizes Allscripts as a Growth and Innovation Leader in the United States Healthcare Data Interoperability Market
Allscripts enables enterprise-level, interoperability-aided EMR workflow adjustments and the subsequent clinical decision-making from a single window SANTA CLARA, Calif., Oct. 7, 2020 — Frost & Sullivan has identified Allscripts, a global healthcare IT company, as a growth and innovation top-performing leader in the Frost Radar™: US Healthcare Data Interoperability Market, 2020. Allscripts demonstrates…