Wearing Fitbit devices with Health2Sync glucose control app clinically proven to help both users and healthcare providers to control and better manage the symptoms of Type 2 diabetes Results from the study in Taiwan revealed an improvement in patients’ health conditions, with participants recording higher levels of physical…
For 2020, The World Innovation Summit for Health (WISH) Delivers Leading Speakers and Compelling Discussions via an Immersive Virtual Platform
DOHA, Qatar, Nov. 9, 2020 — The World Innovation Summit for Health (WISH) returns on 15 November, 2020, offering an impressive range of speakers, networking opportunities, discussions and presentations that explore global health challenges, and – all presented through a purpose-built 3D virtual platform. Oscar-winning actor, humanitarian…
Clarivate Expands International Real-World Data Offering with Addition of Techtrials Brazilian Dataset
Partnership to provide life science, healthcare and research professionals with comprehensive RWD capturing approximately 80% of the Brazilian population LONDON, Nov. 2, 2020 — Clarivate Plc (NYSE:CCC), a global leader in providing trusted information and insights to accelerate the pace of innovation, today announced the…
CaDi introduces Lycofertilic™ – high potency, targeted supplements with algae DHA Omega-3 to provide anti-ageing support for the ovarian reserve and to prepare for egg retrieval and IVF
LONDON, Oct. 23, 2020 — Cambridge Diagnostic Imaging (CaDi) is launching a new generation of targeted daily supplements, LycofertilicTM for fertility support and LycofertilicTM Prime to prepare for egg retrieval and IVF. The ovarian reserve and egg quality of women declines with age. This depletion is exacerbated by stress, imbalanced…
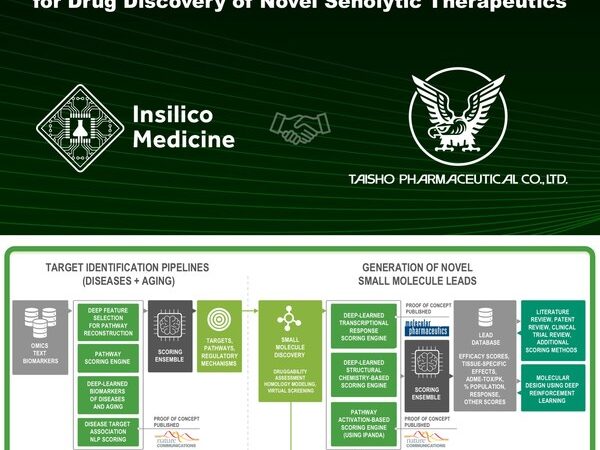
Insilico partners with Taisho on end-to-end AI-powered senolytic drug discovery
HONG KONG, Oct. 15, 2020 — Insilico Medicine announced today that Taisho Pharmaceutical Co., Ltd. and Insilico have entered into a research collaboration to identify novel therapeutics against aging. Insilico Medicine will utilize both the target discovery and generative chemistry parts of its Pharma.AI platform in this collaboration. It will use…
QYNAPSE (France) and TRUE POSITIVE MEDICAL DEVICES (Canada) are partnering to provide the most advanced AI platform for brain diseases
Strategic partnership in brain imaging and AI PARIS, MONTREAL, and QUEBEC CITY, Oct. 13, 2020 — Acquisition of TRUE POSITIVE MEDICAL DEVICES by QYNAPSE A strategic collaboration that covers 15 patents, including 9 issued in the U.S. and Canada A…
Life-changing medical innovations that makes the impossible possible
STOCKHOLM, Oct. 8, 2020 — Medical wire that stops the trembling caused by Parkinson’s disease. Precision tools that can manufacture tailored prosthetic limbs durable and flexible enough to race down the slopes. And 3D-printing technologies that can create scull and spinal implants in just a few hours. With new and…
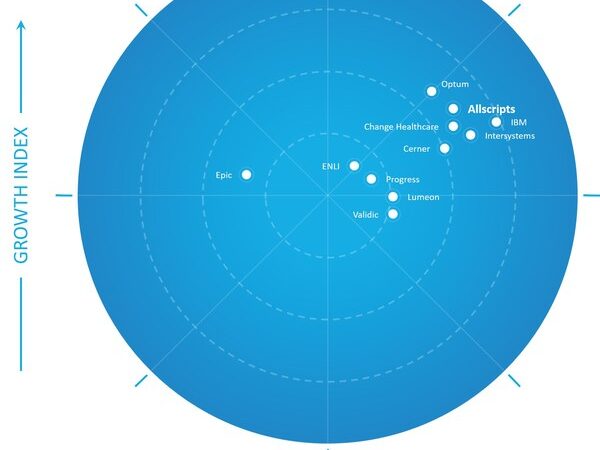
Frost & Sullivan Recognizes Allscripts as a Growth and Innovation Leader in the United States Healthcare Data Interoperability Market
Allscripts enables enterprise-level, interoperability-aided EMR workflow adjustments and the subsequent clinical decision-making from a single window SANTA CLARA, Calif., Oct. 7, 2020 — Frost & Sullivan has identified Allscripts, a global healthcare IT company, as a growth and innovation top-performing leader in the Frost Radar™: US Healthcare Data Interoperability Market, 2020. Allscripts demonstrates…
Visiopharm Announces Scientific Advisory Board
HOERSHOLM, Denmark, Oct. 6, 2020 — Visiopharm®, the Denmark-based leader in artificial intelligence-driven image analysis, tissue mining, and precision pathology has announced the formation of its scientific advisory board (SAB), comprised of industry experts in digital pathology, quality assessment, oncology, and immunology. AI-driven Digital Precision Pathology is still a relatively…
Smartphone-based Solutions Boost the Global Infectious Disease Point-of-Care Testing Market at an Explosive CAGR of 70.2% in 2020
Digital POCT platforms will enable diagnostic testing beyond the clinic, transforming how assay results are gathered and analyzed, finds Frost & Sullivan SANTA CLARA, California, Oct. 2, 2020 — Frost & Sullivan’s recent analysis, Smartphone-based Solutions Spur the Global Infectious Disease Point-of-Care Testing Market, Forecast to 2024, finds that the outbreak…