Verista supports its clients by implementing pioneering services, solutions and technologies, along with its unwavering client commitment and deep domain expertise SAN ANTONIO, Sept. 19, 2023 — Frost &…
World Leading Rehabilitation Expert Prof. Dr. Christopher Gutenbrunner Visits Siyi Intelligence
SHANGHAI, Aug. 18, 2023 — On August 15th, Prof. Dr. Christopher Gutenbrunner, the former chief physician and Head of Department at the Clinic for Rehabilitation Medicine at Hannover…
Mediballoon’s Online Shop is now live, offering a range of medical balloons and tubing for sale. Mediballoon is your best partner for medical device development.
UNION CITY, Calif., Aug. 9, 2023 — Mediballoon, Inc., a Medeologix company established in 2016 specializing in medical balloon design, development, and scale-up production, is excited to announce the…
TraceLink Accelerates DSCSA 2023 Customer Readiness, Supply Chain Digitalization, and Drug Shortage Predictability in Second Quarter of 2023
As the industry prepares for DSCSA 2023 regulations, 50% of the US MAH market is now EPCIS-enabled through the TraceLink B2N Integrate-Once…
Project Matty: Revolutionizing Care for Children with Autism and ADHD Through AI
SINGAPORE, July 24, 2023 — Project Matty, a pioneering digital health solution, has been launched by a dedicated mother of an ADHD-diagnosed child…
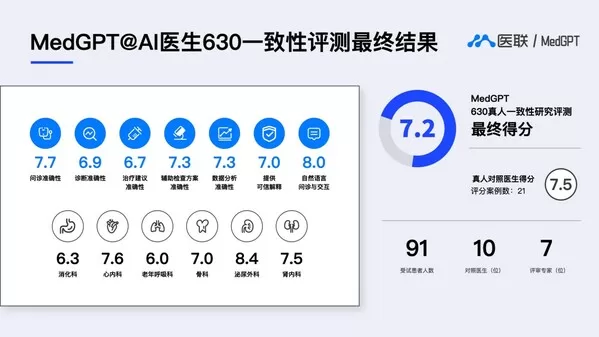
Medlinker’s MedGPT Performs 96% As Well As Top Human Doctors
In a Livestreamed Real-World Assessment, MedGPT’s AI Doctors Rival Diagnostic Accuracy of China’s Leading Doctors BEIJING, July 22, 2023 — Medlinker, a leading digital healthcare company in China, undertook…
Join Taipei City’s Thriving Health Industry: Registration for “Win A Greater Health” Pitch Contest Starts Now!
TAIPEI, July 18, 2023 — The Taipei City Government’s Department of Economic Development is taking proactive steps to attract international startups and foster collaboration with local businesses through its…
Modernising Philanthropy: Kidney Dialysis Foundation Embraces Technology with over 50 GivePlease Donation Terminals Islandwide for Flag Day Fundraising
Changing the fundraising landscape, one donation terminal at a time. SINGAPORE, July 14, 2023 — The Kidney Dialysis Foundation (KDF) is…
Lunit’s AI-Powered Lung Cancer Screening Solution Significantly Affects Radiologists’ Diagnostic Determination – Published in Radiology
– Recent study conducted by Seoul National University Hospital provides strong evidence that high-accuracy AI model improves radiologists’ chest X-ray analysis performance…
United Imaging Introduces Next-Generation PET/CT Systems and Integrated Molecular Technology Platform at SNMMI
With uMI Panorama™, United Imaging Continues to Push the Envelope on Whole-Body System Performance HOUSTON, June 26, 2023 — United Imaging, a global leader in manufacturing advanced medical imaging…