Contract Management Leader Donates INR 15 Lakhs to Renowned Biomedical Research Institute to Test Anti-Tuberculosis (BCG) Vaccine as COVID-19 Treatment PUNE, India, May 19, 2020 /PRNewswire/ — Icertis, the leading provider of enterprise contract management in the cloud, today announced…
FlowForma & MicrotechDPS Join Forces, To Support APAC Region With Process Automation Tools
DUBLIN, May 19, 2020 /PRNewswire/ — FlowForma®, the leading provider of Process Automation tools for Microsoft Office® 365, today announced an exciting new partnership with MicrotechDPS, to deliver the award-winning FlowForma Process Automation tool to organizations in Australia and the greater APAC…
VeChain and I-Dante Partnered to Create Blockchain Enabled Medical Data Management Platform for Healthcare Provider in Cyprus
SHANGHAI, May 18, 2020 /PRNewswire/ — With more than 4.6 million infections, 300 thousand deaths and counting caused by COVID-19, national governments, healthcare systems regardless of region and continent have been scrambling to respond to it, and the pandemic has highlighted…
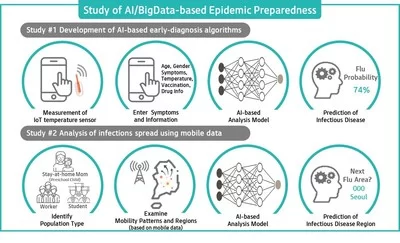
KT announces collaboration on ICT-based Epidemic Preparedness
SEOUL, South Korea, May 17, 2020 /PRNewswire/ — KT Corporation (KRX: 030200; NYSE: KT), South Korea’s largest telecommunications company, announced a three-year research study today funded by the Bill & Melinda Gates Foundation for ICT-based global epidemic response using artificial intelligence (AI)…
Coc Coc Shares Its Contribution in Fighting Against COVID-19 Epidemic
HANOI, Vietnam, May 15, 2020 /PRNewswire/ — Vietnam has made efforts to implement measures of the COVID-19 prevention and achieved positive results. To contribute to this success, businesses, especially technology companies, have taken numerous practical actions. As a leading Vietnamese technology company, Coc…
Frost & Sullivan Assesses the Top 21 Global Risks that Threaten the Next Decade
Inability to mitigate global risks such as rise of infectious diseases like COVID-19 can adversely impact businesses, societies, and economies SANTA CLARA, California, May 14, 2020 /PRNewswire/ — Frost & Sullivan’s recent analysis, Global Future Risks—Future-proofing Your Strategies, 2030, provides a…
Telehealth to Experience Massive Growth with COVID-19 Pandemic, Says Frost & Sullivan
Demand for telehealth will soar by 64.3% in the US in 2020 as the COVID-19 pandemic disrupts the practice of medicine and the delivery of healthcare SANTA CLARA, California, May 14, 2020 /PRNewswire/ — Frost & Sullivan’s recent analysis, Telehealth—A Technology-Based…
Bigo Live Announces ‘Global BIGOer One World Together’ Fundraising Campaign To Support WHO In Fight Against COVID-19
The “Global BIGOer One World Together” fundraising campaign brings together the global BIGO community to raise donations for the World Health Organization’s COVID-19 Solidarity Response Fund Campaign highlights include the release of a music video featuring 100 broadcasters from over…
Leica Biosystems launches Aperio GT 450 DX in Asia enabling high volume clinical labs to scale up digital pathology operations
VISTA, California, May 12, 2020 /PRNewswire/ — Leica Biosystems, the global leader in pathology workflow solutions, announced today that it has launched the Aperio GT 450 DX, its next generation digital pathology scanner, in the APAC region. With continuous loading, no-touch…
111 to Launch First Online Diabetes Patient Management Platform with Lilly China
SHANGHAI, May 8, 2020 /PRNewswire/ — 111, Inc. (“111” or the “Company”) (Nasdaq: YI), a leading integrated online and offline healthcare platform in China, today announced that it is launching a new diabetes patient management platform in cooperation with Lilly…