LONDON, Oct. 23, 2020 — Cambridge Diagnostic Imaging (CaDi) is launching a new generation of targeted daily supplements, LycofertilicTM for fertility support and LycofertilicTM Prime to prepare for egg retrieval and IVF. The ovarian reserve and egg quality of women declines with age. This depletion is exacerbated by stress, imbalanced…
Deep Longevity and Longenesis to Partner on Consent Management Integration and Federated Learning Method Development
HONG KONG, Oct. 23, 2020 — Regent Pacific Group Limited ("Regent Pacific" or the "Company" and together with its subsidiaries, the "Group"; SEHK:0575.HK) today announced that Deep Longevity, Inc,, a company recently acquired by the Group which mainly engaged in the development of explainable artificial intelligence systems to track the rate…
COVID-19 Sparks Boom in Digital Hospitals with Smart Technologies, Improving Quality of Care
Digital hospitals enhance patient care and improve healthcare staff’s efficiency and productivity, finds Frost & Sullivan SANTA CLARA, Calif., Oct. 22, 2020 — Frost & Sullivan’s recent analysis, Digital Hospitals: Creating Growth Opportunities in Patient Care during the COVID-19 Pandemic and Beyond, finds that digital hospitals that deploy smart technologies, such…
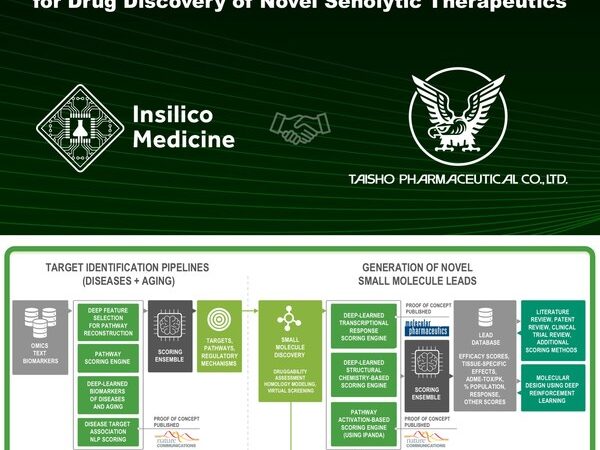
Insilico partners with Taisho on end-to-end AI-powered senolytic drug discovery
HONG KONG, Oct. 15, 2020 — Insilico Medicine announced today that Taisho Pharmaceutical Co., Ltd. and Insilico have entered into a research collaboration to identify novel therapeutics against aging. Insilico Medicine will utilize both the target discovery and generative chemistry parts of its Pharma.AI platform in this collaboration. It will use…
QYNAPSE (France) and TRUE POSITIVE MEDICAL DEVICES (Canada) are partnering to provide the most advanced AI platform for brain diseases
Strategic partnership in brain imaging and AI PARIS, MONTREAL, and QUEBEC CITY, Oct. 13, 2020 — Acquisition of TRUE POSITIVE MEDICAL DEVICES by QYNAPSE A strategic collaboration that covers 15 patents, including 9 issued in the U.S. and Canada A…
“MUSCLE SUIT Every” PR Campaign Selected as “2020 IN2 SABRE Awards Asia-Pacific” Winner, “2020 SABRE Awards Asia-Pacific” Finalist
TOKYO, Oct. 12, 2020 — Innophys Co., Ltd., which develops and sells wearable work-assisting robot MUSCLE SUIT Every, was selected as a finalist in the "2020 SABRE Awards Asia-Pacific," one of the world’s most prestigious PR awards, on September 24 for its publicity campaign to encourage younger people to present their…
BenQ Launches GW2780T Eye-care Monitor and Monitor Light ScreenBar to Protect Consumers’ Eye Health
BenQ’s advanced eye-care technologies and sleek ergonomic designs power the latest work and study setup in a screen-centric world SINGAPORE, Oct. 12, 2020 — BenQ, the world-leading provider of digital lifestyle innovations, today launched the GW2780T Eye-care Monitor and Monitor Light ScreenBar, a powerful combination to safeguard eye health for any…
Beko’s first-of-its kind household product line eliminates more than 99% of bacteria and viruses (including coronavirus)
New home appliances line, launched via a virtual experience, uses UV light technology heat and steam for at-home disinfection ISTANBUL, Oct. 8, 2020 — Beko, Europe’s leading home appliance brand, has developed Hygiene Shield, a ground-breaking portfolio of household products, created in…
Life-changing medical innovations that makes the impossible possible
STOCKHOLM, Oct. 8, 2020 — Medical wire that stops the trembling caused by Parkinson’s disease. Precision tools that can manufacture tailored prosthetic limbs durable and flexible enough to race down the slopes. And 3D-printing technologies that can create scull and spinal implants in just a few hours. With new and…
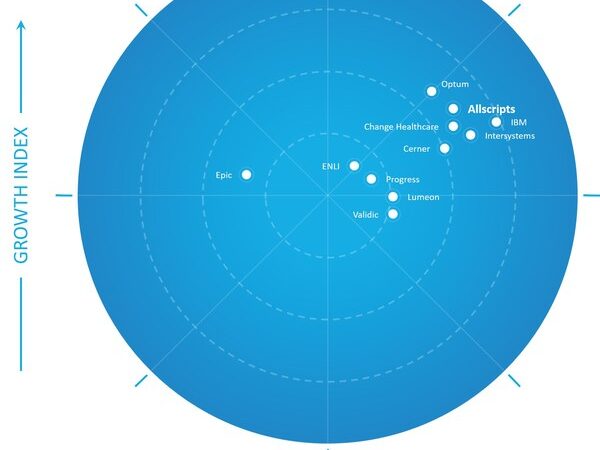
Frost & Sullivan Recognizes Allscripts as a Growth and Innovation Leader in the United States Healthcare Data Interoperability Market
Allscripts enables enterprise-level, interoperability-aided EMR workflow adjustments and the subsequent clinical decision-making from a single window SANTA CLARA, Calif., Oct. 7, 2020 — Frost & Sullivan has identified Allscripts, a global healthcare IT company, as a growth and innovation top-performing leader in the Frost Radar™: US Healthcare Data Interoperability Market, 2020. Allscripts demonstrates…