NANPING, China, June 18, 2021 — Happiness Biotech Group Limited (the "Company" or Nasdaq: HAPP), an innovative China-based nutraceutical and dietary supplements producer and e-commerce services provider, announced today that the Company launched the beta version of official site of Happy Buy International https://www.happybuy.vip, mainly promoting its cross-border e-commerce SaaS…
Reckitt and Shopee support Filipinos in fight against pandemic with ‘Protection Starts Within’ campaign
Reckitt’s first multi-brand regional Super Brand Day on Shopee equips shoppers with health, hygiene, and nutrition tips along with exclusive deals MANILA, Philippines, June 18, 2021 — Reckitt, the global leader in health, hygiene and nutrition, partners with Shopee, the leading e-commerce platform in Southeast Asia and Taiwan to launch its…
Clarivate Report Demonstrates that Nations or Institutions with Diverse Research Priorities Respond More Comprehensively to Unprecedented Scientific Challenges
Forward-looking approach offers a valuable new tool for anticipating and preparing for the unexpected LONDON, June 16, 2021 — Clarivate Plc (NYSE: CLVT), a global leader in providing trusted information and insights to accelerate the pace of innovation, today released a new Global Research…
Medidata Becomes First Company to Offer End-to-End, Unified, Secure Platform for Decentralization of Clinical Trials (DCT)
The first company in the world to unify direct patient data capture technology with study oversight and monitoring, Medidata redefines end-to-end decentralization for sponsors and CROs The unique Medidata Trial Dial™ concept provides the industry’s highest level of customization…
Enamine implements CDD Vault to digitalize its integrated medicinal chemistry, ADME-PK and screening services
The company’s expansion in the biological assay space aided by the data platform SAN FRANCISCO and KYIV, Ukraine, June 10, 2021 — Enamine, a leading provider of R&D Services as well as Screening Compounds, Fragments, Building Blocks and specialized libraries for Drug Discovery, announced…
WhaleTeq Launches First AED Management Platform with DFS200 to Provide Most Effective and Efficient Maintenance Solution
TAIPEI, June 4, 2021 — WhaleTeq, a Taiwan-based Innovative medical device testing solution company, launches the first AED management platform with their latest defibrillator tester, DFS200, to fill in the AED testing and maintenance gap. AED testing and maintenance is of great importance as AED failures can be life-threatening. AED…
VeChain, Together With DNV, Enables Renji Hospital To Launch The World’s First Blockchain-based IVF Service App – MyBaby
SHANGHAI, June 4, 2021 — In partnership with VeChain and DNV, Renji Hospital, a top-ranking hospital in China affiliated with the Shanghai Jiaotong University School of Medicine has announced the launch of smart medical care project – MyBaby, the world’s first blockchain-based In-Vitro…
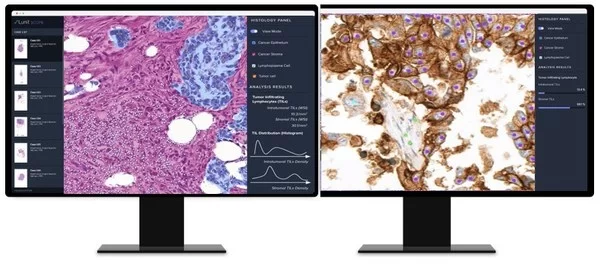
AI-based Analysis of Cancer Tissue Predicts Response to Immunotherapy–Findings to Be Presented at ASCO 2021
Lunit to present four abstracts including one in a ‘Poster Discussion Session’ AI-based tissue analysis platform ‘Lunit SCOPE’ to be launched within the second half of 2021: "We aim to make our AI the new standard for cancer treatment" SEOUL, Korea, June 4, 2021 — A new…
Planetwatch Announces The Listing Of The PLANETS Token On Bitfinex Exchange
ST. GENIS-POUILLY, France, June 3, 2021 — Planetwatch, a French start-up which decentralizes and incentivizes environmental monitoring, announced that its utility token, PLANETS, will be listed on Bitfinex. PLANETS are the first Algorand Standard Asset (barring stablecoins) to be supported on a major…
PolyU develops biomimetic nanosheet for cancer therapy and imaging
HONG KONG, May 31, 2021 — A research team from the Department of Applied Biology and Chemical Technology (ABCT) of The Hong Kong Polytechnic University (PolyU) has developed a novel type of biomimetic nanosheet with a multi-modal imaging function, which can track tumour development and treatment processes in real-time. By…