RIYADH, Saudi Arabia, Dec. 21, 2024 — The Internet Governance Forum (IGF) successfully concluded its 19th edition, hosted in Riyadh at the King Abdulaziz International Conference Center from December…
Hankyung.com introduces: MecKare, Leading the AI-powered Innovation in Health Monitoring Solution
– Leading efficient care management for the elderly with unimpeded smartcare https://img.hankyung.com/pdsdata/pr.hankyung.com/uploads/2024/11/image01-1.png SEOUL, South Korea, Nov. 23, 2024 — JCF Technology is a startup that independently developed ‘MecKare’, a radar…
A Comprehensive Introduction of the oneClick+ antigen platform
SHANGHAI, Oct. 22, 2024 — Sanyou Bio provides oneClick+ antigen one-stop solution, from antigen analysis to personalized preparation, to help tumor immunotherapy research and development. oneClick+ antigen platform is…
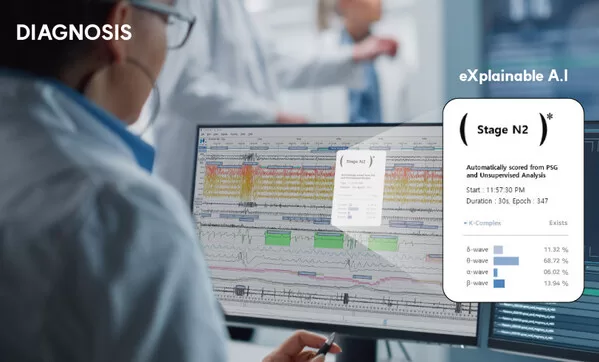
HoneyNaps actively supplies “AI solutions” to U.S. medical institutions
Rapid commercialization and delivery to the U.S. after FDA approval Aiming to achieve a 10% market share within three years Aggressive sales and expansion of distribution network through…
Clarivate and Relatable Healthcare Forge Strategic Partnership to Transform Medtech Sales Contracting Process
Clarivate Medtech Intelligence and Relatable software enhance product data management and competitive conversion efficiency for medtech sales, contracting and pricing teams LONDON,…
CSafe launches reusable Silverpod pallet shipper to help pharma companies save on disposal costs and hit sustainability targets
New pallet improves cost and operational efficiency while reducing environmental impact; includes real-time visibility with cloud connectivity Key features include: Made of fully reusable components and recyclable PCM (phase…
Australasian College of Pharmacy and MedAdvisor Solutions Launch MedAdvisor software training for the Queensland Community Pharmacy Scope of Practice Pilot
Uplifting pharmacists’ role in primary care and positioning pharmacies as essential care destinations in local communities CAMBERWELL, Australia, July 25, 2024 –…
Kangsters Unveils Revolutionary Treadmills at Rollettes Experience 2024: Celebrating Innovation and Empowerment
LOS ANGELES, July 11, 2024 — Kangsters, the pioneering South Korean adaptive tech startup, proudly announces its participation in the Rollettes Experience 2024. This renowned event, now celebrating its…
Kantar BrandZ 2024 Rankings: Haier Leads for the Sixth Consecutive Year as Premier IoT Ecosystem Brand
QINGDAO, China, June 12, 2024 — Haier, the number one brand in the household appliances sector, has once again distinguished itself as the only Internet of Things (IoT) ecosystem…
Orion Health awarded Panda Health Partner status for its Orchestral Health Intelligence Platform
NEW YORK, May 19, 2024 — Orion Health, a global leader in digital health solutions, announced today that it has been awarded partner status by Panda Health for its…